PainTrace® in research can be used for:
- Stratification - Tailored methodology can enhance the validity of your findings.
- Pilot Trials - Strengthen your application and protocol by providing evidence of your study's design and its relevance to addressing pain-related issues. Phase
- Studies - Smaller, well-defined groups can yield significant insights into pain measurement and improve the precision of your findings.
ACHIEVE YOUR GOALS
Book a meeting to discuss PainTrace® in research.
How do we turn PainTrace® data into meaningful insights in research?
PainTrace® is an objective neurobiosignature correlated with human self-reported pain
PainTrace differentiates acute and chronic pain, measuring both magnitude and duration of the pain experience.
Quantify the individual experience of pain with an objective outcome measure to strengthen research conclusions.
The PainTrace® Pain Scale

Interpreting the PainTrace Pain Scale
Baseline Value
The PainTrace Baseline measures chronic pain - positive values indicate degree of non-painful state and increasing negative values indicate mild, moderate, and severe chronic pain state.
Pain Peaks
The PainTrace Acute Peaks quantify acute pain in response to in response to applied stimuli such as examination, movement, or touch. Peak amplitudes are highlighted by intensity.
How do we turn PainTrace® data into meaningful insights in research?
The PainTrace® Pain Scale
PainTrace® is an objective neurobiosignature correlated with human self-reported pain
PainTrace differentiates acute and chronic pain, measuring both magnitude and duration of the pain experience.
Quantify the individual experience of pain with an objective outcome measure to strengthen research conclusions.
![]()
![]()
![]()
![]()


Interpreting the PainTrace Pain Scale
Baseline Value
The PainTrace Baseline measures chronic pain - positive values indicate degree of non-painful state and increasing negative values indicate mild, moderate, and severe chronic pain state.
Pain Peaks
The PainTrace Acute Peaks quantify acute pain in response to in response to applied stimuli such as examination, movement, or touch. Peak amplitudes are highlighted by intensity.
Mild Acute Pain
Moderate Acute Pain
Severe Acute Pain
CASE EXAMPLES
PainTrace® demonstrates reduced pain in 95% of lower back patients post-treatment
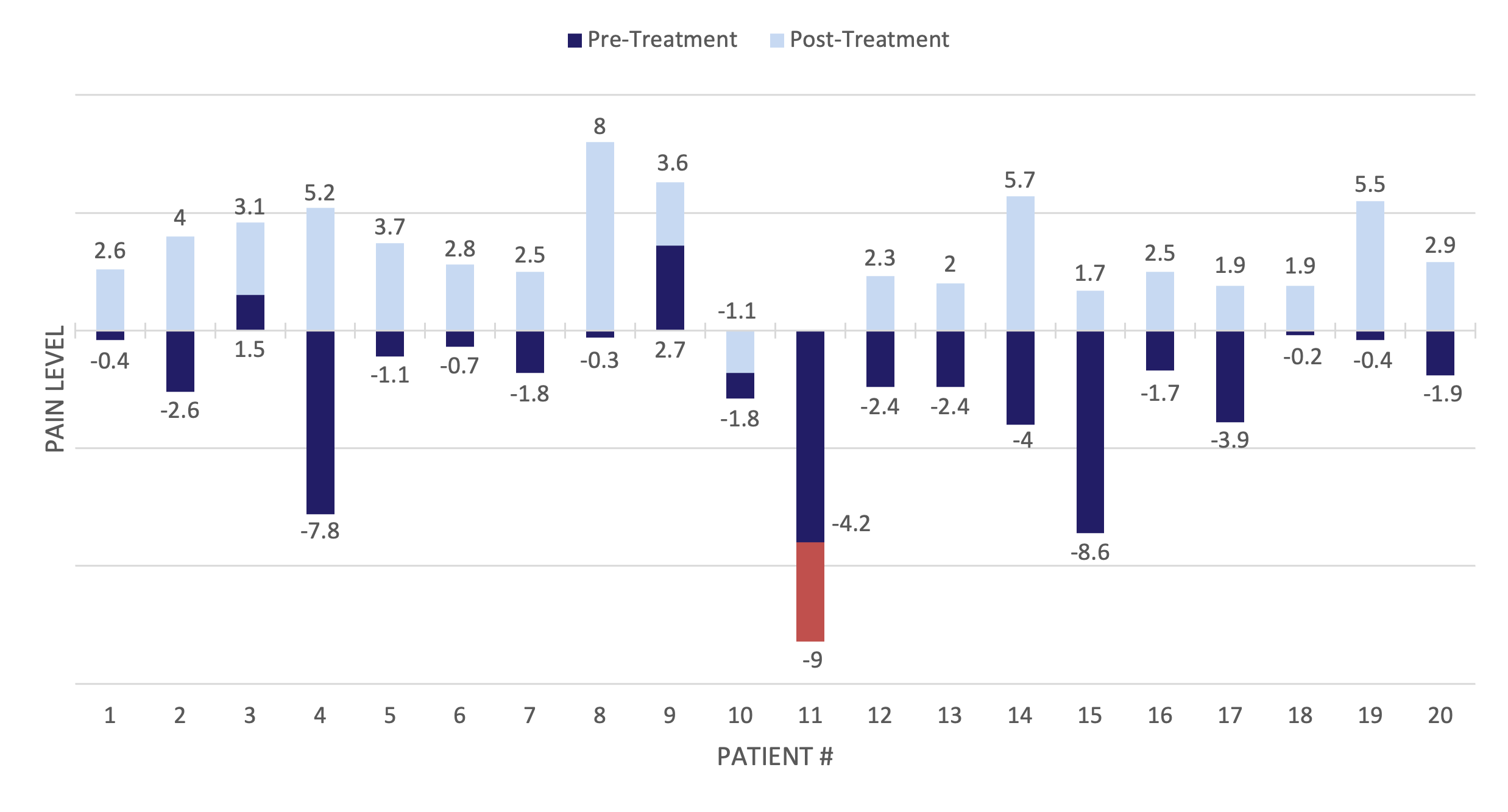
PainTrace® was used to objectively measure pain levels in individuals with chronic lower back pain before and after treatment.
The results showed that 95% of patients experienced less pain, as evidenced by more positive or less negative PainTrace® values post-treatment.
PainTrace® demonstrates reduced pain in 95% of lower back patients post-treatment
PainTrace® was used to objectively measure pain levels in individuals with chronic lower back pain before and after treatment.
The results showed that 95% of patients experienced less pain, as evidenced by more positive or less negative PainTrace® values post-treatment.

PainTrace® provides an objective measure of pain throughout the surgical recovery process
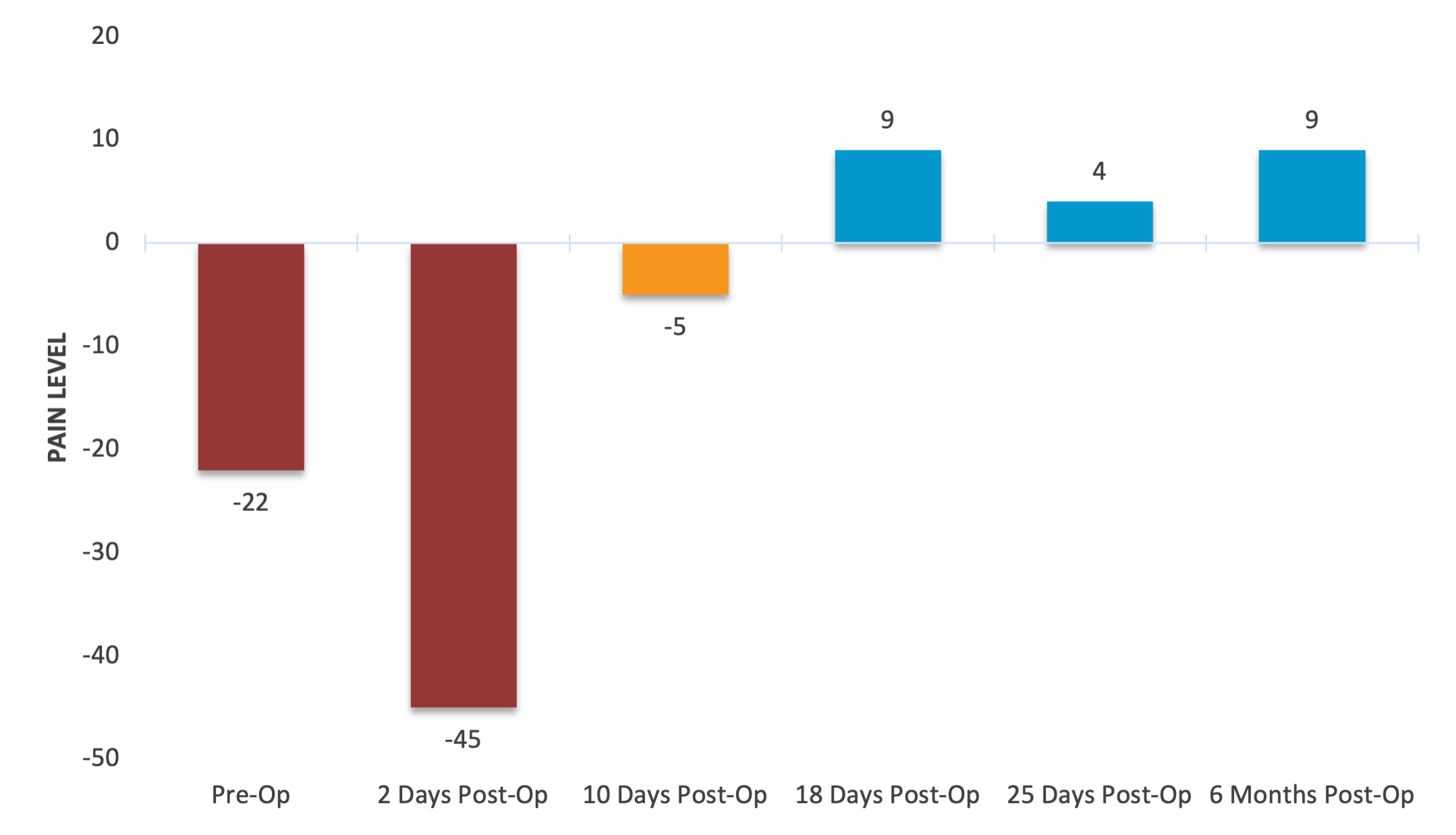
Monitor Pre- and Post-Surgical Pain
Pain levels were recorded before surgery and monitored over time post-operatively. An initial increase in pain signal was observed immediately after surgery, followed by steady improvement. These values reflect reduced pain and support PainTrace® as a tool for tracking surgical recovery and long-term treatment efficacy.
PainTrace® provides an objective measure of pain throughout the surgical recovery process

Monitor Pre- and Post-Surgical Pain
Pain levels were recorded before surgery and monitored over time post-operatively. An initial increase in pain signal was observed immediately after surgery, followed by steady improvement. These values reflect reduced pain and support PainTrace® as a tool for tracking surgical recovery and long-term treatment efficacy.
Chronic Pain with NSAID's for 18 weeks
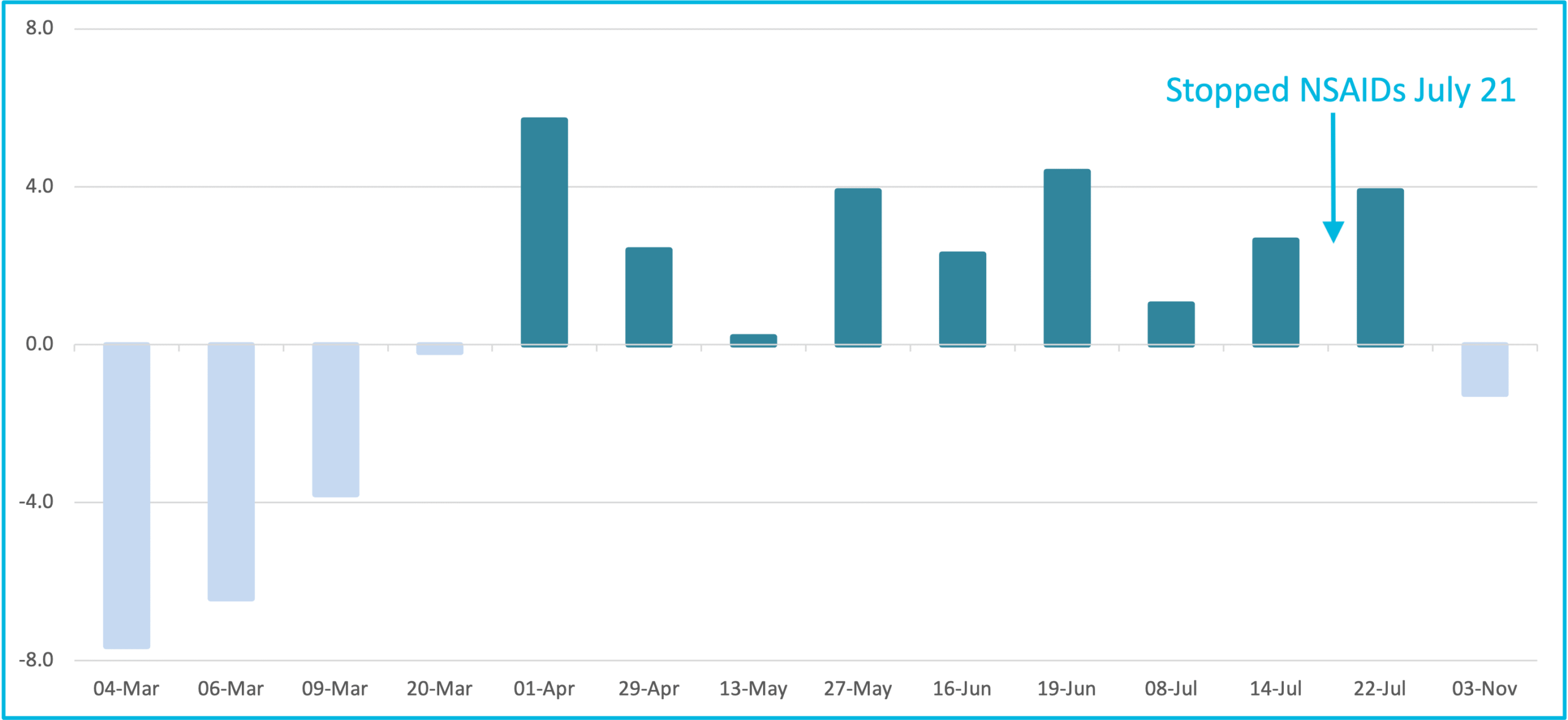
Effect on PainTrace® Baseline values, indicating chronic pain state, with the administration of NSAIDs over 18 weeks
PainTrace can be used to track chronic pain and assess treatment efficacy by comparing baseline averages across the pre-treatment, active treatment, and post-treatment phases. Changes in the baseline average reflect shifts in the chronic pain state, providing objective insight into therapeutic impact.
Chronic Pain with NSAID's for 18 weeks

Effect on PainTrace® Baseline values, indicating chronic pain state, with the administration of NSAIDs over 18 weeks
PainTrace can be used to track chronic pain and assess treatment efficacy by comparing baseline averages across the pre-treatment, active treatment, and post-treatment phases. Changes in the baseline average reflect shifts in the chronic pain state, providing objective insight into therapeutic impact.
“To better understand chronic pain, we first need to address the problem of how to evaluate the severity of pain.”
(Objective Pain Assessment: a Key for the Management of Chronic Pain. F1000 Research)
THE CHALLENGES
THE IMPACT

PainTrace® Partnership Program
2019-01-01
Discovery
Share goals and learn how PainTrace can optimize your research.
2020-01-01
Protocol Review & Consultation
Work with our team and confidentiality to develop the scope of work.
2021-01-01
Study Implementation
PainTrace study resources and comprehensive training. Study kick-off and ongoing support.
2019-01-01
Final Report
PainTrace objective analysis based on study protocol.
PainTrace® Partnership Program
2019-01-01
Discovery
Share goals and learn how PainTrace can optimize your research.
2020-01-01
Protocol Review & Consultation
Work with our team and confidentiality to develop the scope of work.
2021-01-01
Study Implementation
PainTrace study resources and comprehensive training. Study kick-off and ongoing support.
2019-01-01
Final Report
PainTrace objective analysis based on study protocol.
Use PainTrace® in Your Research Protocol!
Contact us to find out how PainTrace impacts your research goals.
